99 %
OTIF
On-time, in-full (last 90 days)
On-time, in-full (last 90 days)
1 %
EXCURSIONS
In-route excursions on qualified lanes
In-route excursions on qualified lanes
5
GDP NODES
Qualified global network
Qualified global network
100 %
VISIBILITY
Real-time temp + custody + audit trail
Real-time temp + custody + audit trail
Built into every service: shipping, packaging, and storage.
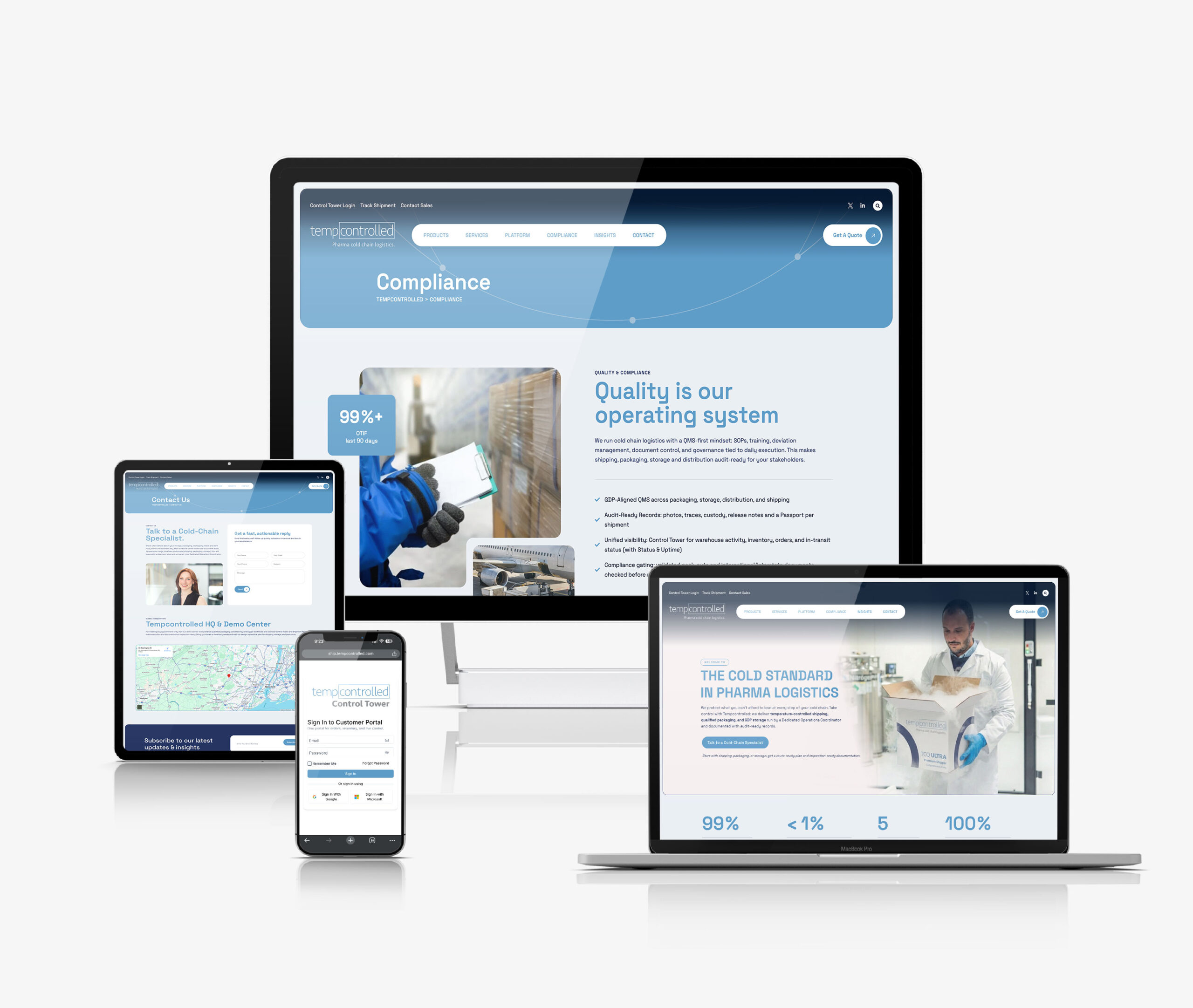
Audit-ready quality & compliance
Quality isn’t a department, it’s how we run the operation. We apply GDP-compliant processes, training, and controls end-to-end: conditioned pack-outs, monitored storage, and in-transit custody and temperature tracking. Every job generates the evidence you need for inspections and investigations without last-minute scramble.

Quality System
GDP-aligned QMS with SOPs, CAPA, change control, vendor qualification, and role-based training across shipping, packaging, and storage.
Real-Time Visibility
Live temperature, location, and inventory status in warehouse and in transit; predictive ETAs plus conditioning and excursion alerts.
Regulatory Readiness
Document validation and pre-clearance, labeling/HTS support, site qualification, and lane-risk checks to reduce holds and deviations.
Audit-Ready Records
Inspection-ready documentation with custody chain, conditioning logs, storage environment data, temperature traces, milestones, and deviations/CAPA, searchable and shareable.
Expert-led execution, documented for QA
In-range, on-time cold-chain logistics for biotech & pharma
From clinical kits to commercial lots, we design the lane, condition the pack-out, manage GDP storage, and execute shipping door-to-door anywhere in the world. Real-time temperature, location, and exception workflows keep teams ahead of risk. Every shipment is documented with a shareable Shipment Passport for inspection-ready proof.
{"cpt":"service","style":"1","columns":"3","show":5,"order":"DESC","orderby":"none"}
01
Clinical & Commercial
From R&D to commercial, all temps and sizes, designed for in-range, on-time delivery.
02
Direct-to-Patient
Site and home deliveries with identity checks, custody controls, and monitored returns.
03
Laboratory Logistics
Specimens, reagents, instruments, and calibrations with documented handling.
05
Storage & Distribution
GDP storage (2–8 °C, CRT, frozen), inventory control, pick/pack, site distribution.
Tempcontrolled Control Tower
One operating system for your cold chain
Shipping, packaging, and storage work best when they’re run together. We combine lane planning, SOP-driven execution, and proactive exception management, then deliver inspection-ready documentation so Ops and QA stay aligned.
100
%
Systems
Operational
100
%
API Uptime
Last 90 Days
- Warehouse Management — Lot/expiry and temp-zone visibility, receive/put-away, conditioning, pick/pack, QA release, fully traceable.
- Order Management — Request shipments; we design the lane and pack-out, manage milestones, and handle each move door-to-door, including regulatory docs/pre-clearance.
- Visibility & Alerts — Live temperature, location, inventory status, predictive ETAs, and excursion/risk signals.
- Shipment Passport — Shipping documents, custody chain, conditioning logs, temperature data, milestones, all downloadable and shareable.
- Integrations & Security — APIs to your systems, read-only Shipment Passport links for QA/auditors; GDP-aligned workflows, role-based access, change control.
{"cpt":"client","style":"1","columns":"6","show":9,"order":"DESC","orderby":"none"}
Client-01
Client-02
Client-03
Client-04
Client-05
Client-06
Client-07
Client-08
Client-09
We connect you to the world
Qualified GDP Storage & Distribution Network
GDP storage with monitored environments, scan-based workflows, and disciplined handoffs, coordinated by Tempcontrolled as your single point of accountability.
5
GDP locations
830
K
Sq. feet of temperature controlled storage
99.9
%
On-time order dispatch (last 90 days)
Tempcontrolled Qualified (TCQ) Packaging
Lane-matched choices that survive the route, not just the lab.
Tempcontrolled Qualified (TCQ) is our qualified packaging line: ISTA-qualified shippers and documented pack-out configurations executed through approved SOPs, defined lane assumptions, and documented handling.
Enhance Your Operations
Our unique model aligns our team
to your business
-
 01
01Accountability
A Dedicated Operations Coordinator handles your needs, end-to-end. -
 02
02Expertise
Exception playbooks, escalation discipline, audit-ready documentation. -
 03
03Better Operations
Less firefighting, more value-creating work for your team.



Pre-clinical
Clinical trials
Commercial transport
Direct-to-patient
Laboratory logistics
Qualified packaging
Storage & Distribution
Cold Chain News, Guidance, & Playbooks
Pharma Logistics Briefing
{"cpt":"blog","style":"2","show":5,"order":"DESC","orderby":"date"}
Welcome to the new Tempcontrolled.com
Welcome to the new Tempcontrolled.com! Tempcontrolled provides pharma cold…
Tempcontrolled Team
1 Feb, 2026
Contact Us
Talk to a Cold Chain Specialist.
Share a few details about your storage, packaging, or shipping needs and we’ll schedule a brief intake call to confirm lanes, temperature range, timelines, and scope (shipping, packaging, storage). You will leave with a clear next step and an owner: your Dedicated Operations Coordinator.










